Last Updated on 29/01/2026 by James Anderson
Navigating Daily Modafinil Administration
The central question of daily modafinil use requires a nuanced, evidence-based examination that considers pharmacological mechanisms, individual risk profiles, and long-term health outcomes. Through my clinical experience and review of current literature, I’ve developed a framework for evaluating daily administration that prioritizes safety and sustainability. We will explore the neurochemical foundations, develop a comprehensive risk-assessment protocol, and provide actionable strategies for both clinical and off-label contexts.
Neuropharmacological Foundations of Chronic Administration
1. The Unique Pharmacology of Modafinil: Beyond Simple Stimulation
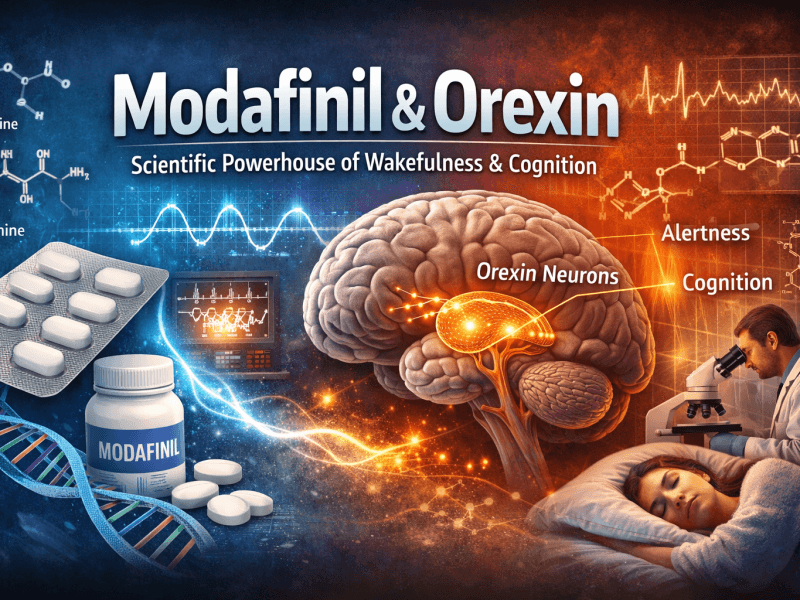
Modafinil’s classification as a eugeroic (wakefulness-promoting agent) rather than a traditional stimulant is crucial to understanding its long-term use profile. The drug operates through several complementary mechanisms:
Primary Mechanism: Selective Dopamine Modulation
Modafinil’s principal action involves the selective inhibition of dopamine reuptake through binding to the dopamine transporter (DAT). Unlike amphetamines that cause non-selective vesicular dopamine release, modafinil’s action is primarily presynaptic, leading to increased dopamine availability in specific brain regions without widespread neuronal excitation. This targeted approach results in:
- Enhanced signaling in prefrontal cortical areas responsible for executive function
- Moderate activation of striatal pathways involved in motivation and reward
- Minimal impact on peripheral sympathetic pathways compared to traditional stimulants
Secondary Neurotransmitter Systems
Current research indicates modafinil influences multiple neurotransmitter systems:
- Orexin/Hypocretin Activation: Modafinil stimulates orexin-producing neurons in the lateral hypothalamus, promoting sustained wakefulness without disrupting normal sleep architecture when properly dosed
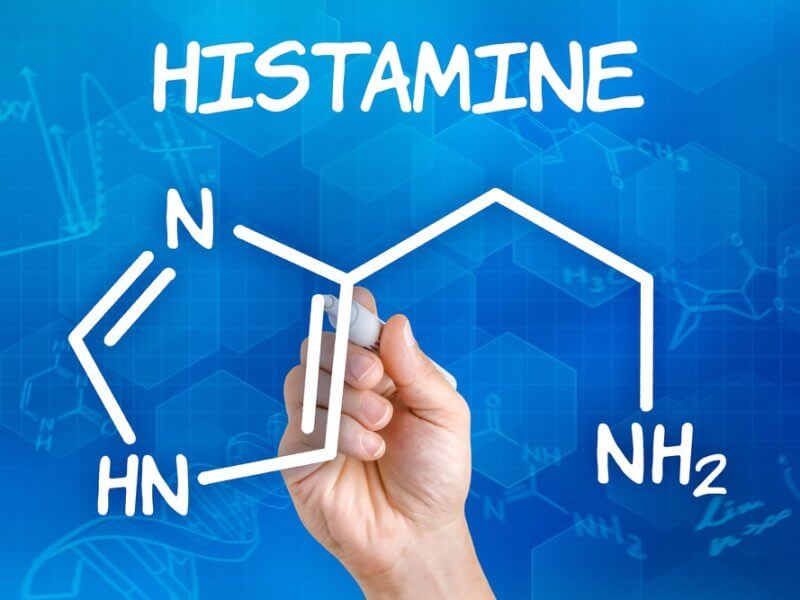
- Histaminergic Engagement: The drug increases histamine release in the tuberomammillary nucleus, contributing to cortical activation and alertness
- GABAergic Modulation: Evidence suggests modafinil may reduce GABA-mediated inhibition in key arousal centers, though this mechanism requires further elucidation
- Noradrenergic Effects: Modafinil elevates extracellular norepinephrine in the hypothalamus and other wake-promoting regions
2. Pharmacokinetics of Long-Term Administration
Understanding modafinil’s metabolic pathways is essential for predicting interactions and long-term effects:
Metabolic Pathway Considerations
- CYP3A4 Metabolism: Approximately 60% of modafinil metabolism occurs via cytochrome P450 3A4, creating potential interactions with medications that induce or inhibit this pathway
- Autoinduction Potential: Unlike many substances metabolized by CYP3A4, modafinil exhibits minimal autoinduction, meaning it doesn’t significantly accelerate its own metabolism with prolonged use
- Elimination Half-Life: The 12-15 hour half-life allows once-daily dosing but contributes to accumulation if dosed too frequently, particularly with the armodafinil enantiomer
- Active Metabolites: Modafinil sulfone and modafinil acid are pharmacologically active metabolites with extended half-lives that may contribute to effects during chronic administration
Individual Metabolic Variants
Genetic polymorphisms in CYP enzymes create substantial interindividual variability in modafinil metabolism, necessitating personalized dosing strategies. Approximately 15-20% of the population are intermediate or poor metabolizers through CYP3A4, potentially experiencing heightened effects or prolonged half-life.
Comprehensive Risk Assessment for Daily Use
1. Tolerance Development: Mechanisms and Management Strategies
The potential for tolerance development represents one of the most significant concerns in long-term modafinil use. Current understanding suggests tolerance manifests differently across various domains:
Neuroadaptive Responses
- Dopamine Receptor Downregulation: Chronic DAT inhibition may lead to compensatory downregulation of post-synaptic dopamine receptors, particularly D2 receptors in striatal regions
- Transporter Upregulation: Some evidence suggests increased DAT expression as a homeostatic response to chronic reuptake inhibition
- Signal Transduction Adaptations: Alterations in intracellular signaling cascades downstream of dopamine receptors may contribute to reduced perceived efficacy over time
Clinical Management of Tolerance
- Strategic Cycling Protocols: Implementing structured periods of abstinence (drug holidays) can help restore receptor sensitivity. Recommended approaches include:
- 5:2 protocol (5 days on, 2 consecutive days off weekly)
- 4-week cycles (3 weeks on, 1 week off)
- Weekend-only cessation for weekday users
- Dose Optimization: Rather than indefinite dose escalation, maintaining the minimum effective dose reduces tolerance risk. The principle of “dose holiday” (briefly reducing to 50% of maintenance dose for 3-5 days) can restore responsiveness without complete cessation.
- Adjunctive Interventions: Incorporating non-pharmacological cognitive enhancers (exercise, mindfulness training, nutritional optimization) can reduce reliance on pharmacological agents while maintaining performance.
2. Cardiovascular Safety Profile
Long-term cardiovascular effects require careful monitoring, particularly in individuals with pre-existing conditions or cardiovascular risk factors:
Blood Pressure Considerations
Modafinil typically produces mild increases in systolic (3-7 mmHg) and diastolic (2-5 mmHg) blood pressure. These effects are generally dose-dependent and most pronounced during initial weeks of treatment. However, several factors modulate cardiovascular response:
- Caffeine Interaction: Concurrent caffeine consumption potentiates modafinil’s pressor effects, creating a synergistic increase in blood pressure
- Individual Variability: Approximately 10-15% of users experience more substantial blood pressure elevation (>10 mmHg systolic), often related to individual sympathetic tone
- Time-Dependent Effects: Cardiovascular stimulation typically peaks 2-4 hours post-administration and gradually declines with the drug’s elimination
Monitoring Protocol for Long-Term Users
- Baseline Assessment: Pre-treatment evaluation should include resting blood pressure, heart rate, and ideally, ambulatory blood pressure monitoring if hypertension is suspected
- Regular Monitoring Schedule:
- Weeks 1-4: Blood pressure assessment 2-3 times weekly at consistent times relative to dosing
- Months 2-6: Weekly measurements
- Beyond 6 months: Biweekly to monthly assessments, depending on stability
- Red Flag Parameters: Discontinuation or dose reduction should be considered with:
- Sustained systolic pressure >140 mmHg or diastolic >90 mmHg
- Heart rate consistently >100 bpm at rest
- Development of arrhythmias or palpitations
3. Endocrine and Metabolic Considerations
Emerging research suggests modafinil may influence several endocrine axes, though clinical significance remains uncertain:
Hypothalamic-Pituitary-Adrenal (HPA) Axis
Modafinil exhibits complex interactions with stress-response systems:
- Acute Effects: Single doses typically increase cortisol and ACTH secretion, consistent with its arousal-promoting properties
- Chronic Adaptation: With prolonged administration, HPA axis responses generally normalize, though individual variability exists
- Clinical Implications: Users with pre-existing HPA dysregulation (adrenal insufficiency, Cushing’s syndrome) should exercise particular caution
Reproductive Hormone Interactions
Limited evidence suggests potential effects on sex hormone regulation:
- Prolactin Modulation: Modafinil may mildly suppress prolactin secretion, though typically within normal physiological ranges
- Gonadotropin Effects: Animal studies suggest possible interactions with gonadotropin-releasing hormone (GnRH) signaling, but human data remains insufficient
- Practical Recommendations: Individuals with endocrine disorders affecting reproductive function should undergo baseline and periodic hormone assessment if using modafinil long-term
Clinical Protocol for Long-Term Administration
1. Initiation and Titration Phase (Weeks 1-8)
A structured approach to treatment initiation minimizes adverse effects while establishing therapeutic efficacy:
1-2 Week: Initial Assessment and Low-Dose Initiation
- Starting Dose: 50-100 mg administered upon waking, preferably with food to minimize gastrointestinal discomfort
- Monitoring Parameters: Daily tracking of subjective effects, side effects, sleep quality, and vital signs (morning and evening)
- Response Assessment: Document specific cognitive domains improved (working memory, sustained attention, task switching) versus unaffected areas
3-4 Week: Dose Optimization
- Dose Adjustment Criteria: Consider increasing to 100-200 mg daily only if:
- Initial response is subtherapeutic
- Side effects remain minimal or manageable
- Sleep architecture remains preserved (confirmed by sleep journaling)
- Split-Dosing Strategy: For individuals requiring all-day coverage, consider 100 mg upon waking + 50-100 mg at noon (not after 2 PM to protect sleep)
5-8 Week: Consolidation and Habit Formation
- Stable Regimen Establishment: Maintain consistent timing and conditions of administration
- Lifestyle Integration: Begin incorporating non-pharmacological cognitive support strategies
- Comprehensive Review: Assess overall benefits versus burdens, considering both quantitative metrics (productivity measures) and qualitative experiences
2. Maintenance Phase Protocols
Successful long-term use requires structured maintenance strategies:
Cyclical Dosing Framework
Empirical evidence supports several cycling approaches:
- Weekly Cycling: 5 consecutive days of use followed by 2 complete days of abstinence
- Monthly Cycling: 21-25 days of use followed by 5-7 day washout period
- Quarterly Cycling: 8-10 weeks of use followed by 2-3 week extended break, particularly valuable for reassessing baseline functioning
Periodic Comprehensive Evaluation
Every 3-6 months, conduct a structured review including:
- Objective cognitive assessment using validated tools (Digit Symbol Substitution Test, Stroop Test)
- Quality of life and functional impairment measures
- Review of medication and supplement regimen for interactions
- Cardiovascular and metabolic health reassessment
3. Discontinuation and Tapering Strategies
Although modafinil isn’t associated with classic withdrawal syndromes, structured discontinuation prevents rebound phenomena:
Gradual Tapering Protocol
- Step 1: Reduce dose by 25-50% for 5-7 days
- Step 2: Further reduce to 25% of maintenance dose or transition to alternate-day dosing for 7 days
- Step 3: Complete discontinuation with implementation of supportive strategies
Supportive Measures During Discontinuation
- Sleep Optimization: Implement strict sleep hygiene, consider short-term melatonin or magnesium supplementation if sleep disruption occurs
- Cognitive Support: Increase engagement in physical exercise, mindfulness practices, and structured task management
- Energy Management: Schedule demanding cognitive tasks during natural circadian peaks, typically late morning for most individuals
Special Populations and Contexts
1. Age-Related Considerations
Young Adults (18-25)
The developing prefrontal cortex may respond differently to chronic modafinil exposure:
- Enhanced neuroplasticity may increase both potential benefits and vulnerabilities
- Greater propensity for risk-taking behaviors necessitates careful monitoring of dosing compliance
- Educational or occupational demands should be weighed against long-term neurobiological considerations
Middle-Aged Adults (40-65)
This population often exhibits different pharmacokinetics and risk profiles:
- Reduced hepatic and renal clearance may increase drug accumulation
- Higher prevalence of comorbid conditions (hypertension, sleep apnea) requires comprehensive evaluation
- Potential benefits for age-related cognitive decline must be balanced against cardiovascular risks
Geriatric Population (>65)
Limited data exists for this demographic, necessitating extreme caution:
- Start with significantly reduced doses (25-50 mg daily)
- Enhanced monitoring for drug interactions due to increased medication burden
- Particular attention to cardiovascular parameters and fall risk
2. Comorbid Conditions Requiring Special Consideration
Psychiatric Comorbidities
Modafinil’s effects vary substantially across psychiatric conditions:
- Depressive Disorders: May provide beneficial activation but risk exacerbating anxiety or agitation
- Bipolar Spectrum: Contraindicated in active mania; requires mood stabilizer coadministration in euthymic patients
- Anxiety Disorders: May improve cognitive symptoms but potentially exacerbate somatic anxiety
- ADHD: Often used off-label with generally favorable responses, though distinct from traditional stimulants
Neurological Conditions
- Migraine Disorders: Modafinil may trigger or exacerbate migraines in susceptible individuals; requires careful monitoring
- Epilepsy: Generally safe in well-controlled epilepsy, but requires neurologist supervision
- Neurodegenerative Conditions: Emerging research in conditions like Alzheimer’s shows mixed results; not currently recommended outside clinical trials
Integrative Approaches and Adjunctive Strategies
1. Nutritional Support for Chronic Users
Specific nutritional strategies can enhance modafinil’s benefits while mitigating side effects:
Mitigating Common Side Effects
- Headache Prevention: Adequate hydration (minimum 2L daily), magnesium supplementation (200-400 mg glycinate), and consistent electrolyte intake
- Gastrointestinal Comfort: Administration with food, particularly containing healthy fats, can reduce nausea; probiotic supplementation may help maintain gut health
- Anxiety Modulation: L-theanine (100-200 mg) can counteract potential anxiety without reducing alertness; omega-3 fatty acids support neuronal membrane stability
Cognitive Support Nutrients
- Cholinergic Support: Alpha-GPC (300-600 mg) or citicoline (250-500 mg) may synergize with modafinil’s dopaminergic effects
- Mitochondrial Support: Coenzyme Q10 (100-200 mg), acetyl-L-carnitine (500-1000 mg), and pyrroloquinoline quinone (PQQ) support neuronal energy metabolism
- Antioxidant Protection: N-acetylcysteine (600-1200 mg) and astaxanthin (4-12 mg) may provide neuroprotection during long-term use
2. Lifestyle Optimization Framework
Non-pharmacological approaches substantially influence long-term outcomes:
Sleep-Wake Cycle Regulation
- Consistent sleep schedule (±30 minutes) even on non-dosing days
- Strategic light exposure (bright morning light, limited blue light after dusk)
- Temperature regulation for optimal sleep initiation (cool bedroom environment)
Physical Activity Integration
- Aerobic exercise (150 minutes weekly) enhances modafinil’s cognitive benefits
- Resistance training improves sleep quality and hormonal regulation
- Movement breaks during sedentary periods potentiate alertness effects
Cognitive Training and Environmental Design
- Dedicated practice of working memory and attention tasks on both dosing and non-dosing days
- Environmental modifications to reduce cognitive load (organized workspace, distraction minimization)
- Implementation of productivity systems that complement rather than depend on pharmacological enhancement
Legal, Ethical, and Future Considerations
1. Regulatory Landscape and Prescription Considerations
The legal status of modafinil varies substantially across jurisdictions, with important implications for long-term users:
- Schedule IV Controlled Substance (United States): Requires prescription, with refill limitations and potential pharmacy scrutiny
- Prescription-Only Medicine (United Kingdom, Canada, Australia): Legal with prescription but commonly used off-label
- Unregulated or Gray Market Status (Many Asian countries): Often available without prescription but with quality control concerns
- Customs and Importation Regulations: Personal importation allowances vary; some countries permit 3-month personal supply while others prohibit any importation without license
Ethical Prescribing Practices
Medical professionals face ethical dilemmas when considering long-term off-label prescribing:
- Informed Consent Requirements: Must include discussion of limited long-term safety data in healthy populations
- Periodic Benefit-Risk Reassessment: Documentation of ongoing therapeutic need and absence of harm
- Alternative Consideration: Exploration of and referral to non-pharmacological interventions
2. Emerging Research and Future Directions
Several promising research avenues may clarify modafinil’s long-term profile:
- Neuroimaging Studies: Longitudinal fMRI investigations of chronic users to identify structural and functional adaptations
- Genetic Research: Pharmacogenomic studies to identify predictors of optimal response and adverse effect susceptibility
- Combination Approaches: Investigation of modafinil combined with other interventions (cognitive training, neurofeedback) for synergistic effects
- Disease-Specific Applications: Expanded research in traumatic brain injury, chemotherapy-related cognitive impairment, and other neurological conditions
Conclusion and Final Recommendations
After extensive review of available evidence and clinical experience, I’ve developed these final recommendations for those considering or currently engaged in daily modafinil use:
For Individuals with Valid Medical Indications:
Daily modafinil represents a well-established, evidence-based treatment when:
- Prescribed and monitored by a knowledgeable physician
- Accompanied by regular medical evaluation including cardiovascular assessment
- Integrated with appropriate management of the underlying sleep disorder
- Periodically reevaluated for continued necessity
For Healthy Individuals Considering Off-Label Use:
A more conservative approach is warranted:
- Exhaust Non-Pharmacological Approaches First: Optimize sleep, nutrition, exercise, and cognitive training before considering pharmacological intervention
- Implement Strict Cycling Protocols: Never use daily without breaks; the 5:2 weekly schedule represents a reasonable maximum frequency
- Establish Clear Boundaries: Define specific use cases and avoid mission creep into general well-being or recreational enhancement
- Maintain Regular Off-Periods: Extended breaks (2-4 weeks quarterly) are essential to assess baseline functioning and prevent psychological dependence
- Monitor Holistically: Track not just cognitive performance but also sleep quality, mood stability, interpersonal relationships, and overall life satisfaction
The decision to use modafinil daily represents a significant commitment to ongoing self-monitoring and medical oversight. When approached with appropriate caution, structured protocols, and respect for the substance’s pharmacological properties, some individuals may find sustained benefits. However, the absence of definitive long-term safety data in healthy populations necessitates a precautionary approach, with regular reevaluation of whether benefits continue to justify potential risks.
FAQ
Q: Can I develop a physical addiction to modafinil if I take it daily?
A: Modafinil has a very low potential for physical dependence according to WHO classification and clinical studies. Discontinuation after long-term use typically results in a return to baseline sleepiness or fatigue rather than a classic withdrawal syndrome. However, psychological habituation the belief that you cannot function optimally without it represents a more significant risk with daily use.
Q: How long can I safely take modafinil daily without a break?
A: For medical conditions like narcolepsy, daily use can continue indefinitely under medical supervision with appropriate monitoring. For off-label cognitive enhancement, evidence-based protocols suggest implementing breaks at minimum weekly (5 days on, 2 days off) with longer breaks (2-4 weeks) every 3-4 months to reassess baseline functioning and prevent tolerance.
Q: Will daily use damage my brain or cognitive function long-term?
A: Current evidence from studies of patients with sleep disorders using modafinil daily for years shows no significant cognitive decline and generally good tolerability. However, these studies involve medically supervised use in populations with specific conditions. There is insufficient long-term data on daily use in otherwise healthy individuals to definitively rule out subtle long-term effects.
Q: What is the single most important monitoring parameter for daily users?
A: Blood pressure represents the most critical objective parameter to monitor regularly. Modafinil can cause modest increases in blood pressure and heart rate, particularly during initial weeks. Users should establish a baseline before starting and monitor at least weekly during the first month, then monthly thereafter. Sustained elevations above 140/90 mmHg warrant dose reduction or discontinuation.
‼️ Disclaimer: The information provided in this article about modafinil is intended for informational purposes only and is not a substitute for professional medical consultation or recommendations. The author of the article are not responsible for any errors, omissions, or actions based on the information provided.
References:
- Outhoff K. Cognitive enhancement: a brief overview. S Afr Fam Pract 2016
- Bard I, Singh I. What should we do about student use of cognitive enhancers? An analysis of current evidence. Neuropharmacol. 2013
- Lanni C, Lenzken SC, Pascale A, Del Vecchio I, Racchi M, Pistoia F, et al. Cognition enhancers between treating and doping the mind. Pharmacol Res. 2008
- Wiegel C. Cognitive test anxiety and cognitive enhancement: the influence of students’ worries on their use of performance enhancing drugs. Subst Use Misuse. 2013
- Young-Powell A, Page L. One in five students have taken the study drug modafinil. The Guardian. 2018
- Kinman BA, Armstrong KJ, Hood KB. Perceptions of Risks and Benefits Among Nonprescription Stimulant Consumers, Diverters, and Non Users. Subst Use Misuse. 2017
- Petersen MA, Norgaard LS, Traulsen JM. Pursuing pleasures of productivity: university students’ use of prescription stimulants for enhancement and the moral uncertainty of making work fun. Cult Med Psychiatry. 2015
- FDA Website – https://www.fda.gov/