Introduction to Modafinil and Its Clinical Role
Modafinil, marketed under the brand name Provigil, is a wakefulness-promoting agent that has gained recognition for its ability to improve alertness and reduce excessive sleepiness. Approved by the FDA in 1998, it is primarily prescribed for sleep-related disorders. Unlike traditional stimulants such as amphetamines, modafinil operates differently in the brain, making it unique in its class.
The Schedule IV classification of Provigil reflects its balanced standing: it carries a recognized medical value but also a controlled potential for abuse. To fully appreciate this classification, it is necessary to explore its pharmacology, clinical applications, and patterns of misuse.
The Pharmacological Profile of Modafinil
Mechanism of Action
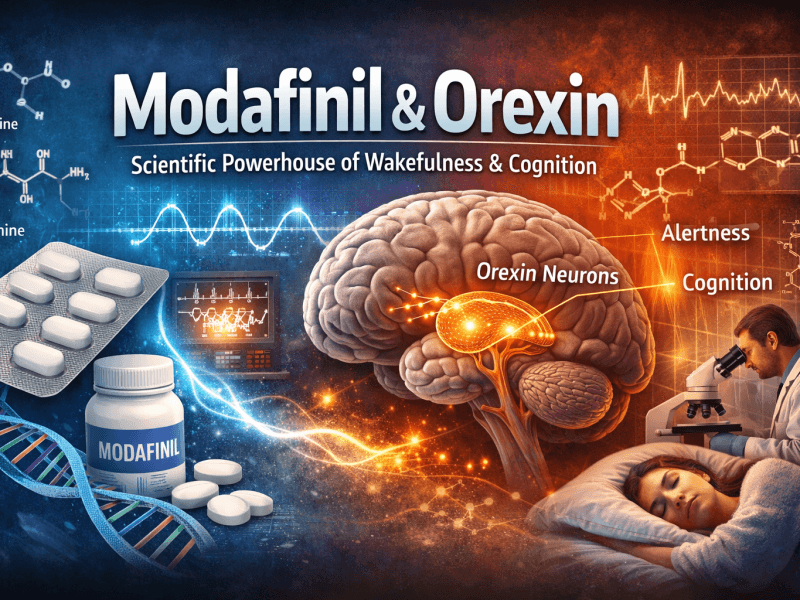
Understanding how modafinil works helps explain why it differs from stronger stimulants. While the full mechanism remains under investigation, researchers have identified its influence on dopaminergic pathways. Unlike amphetamines, modafinil binds weakly to dopamine transporters, producing only a modest increase in dopamine levels. This is key to its lower abuse potential.
Additionally, modafinil appears to activate the orexin (hypocretin) system, which regulates wakefulness and appetite. This makes it particularly effective for sleep disorders while minimizing the “wired” feeling associated with stimulant use. Other neurotransmitters such as histamine and norepinephrine also play a role, contributing to sustained alertness.
In essence, modafinil works as a wakefulness-promoting agent rather than a classical stimulant. Its pharmacological fingerprint explains why it falls under Schedule IV rather than a stricter category.
Medical Uses of Modafinil
Narcolepsy Treatment
Narcolepsy can be profoundly disabling. Patients often experience excessive daytime sleepiness, cataplexy, sleep paralysis, and fragmented nighttime rest. Clinical trials have demonstrated that modafinil improves wakefulness scores and reduces the number of unintentional sleep episodes. Unlike older stimulants, patients report fewer side effects, making modafinil a first-line therapy for narcolepsy.
Obstructive Sleep Apnea (OSA)
For individuals with OSA, continuous positive airway pressure (CPAP) remains the gold standard. However, some patients continue to feel drowsy even after successful CPAP use. Modafinil is prescribed in these cases to alleviate residual sleepiness, enabling patients to function more effectively during the day.
Shift Work Sleep Disorder (SWSD)
Modern society increasingly relies on workers with nontraditional schedules. Healthcare professionals, pilots, and industrial workers often endure irregular shifts that impair circadian rhythms. Clinical studies show modafinil improves reaction times, alertness, and overall productivity in these populations.
Off-Label Cognitive Enhancement
Perhaps the most controversial aspect of modafinil is its off-label use as a cognitive enhancer. Students preparing for exams, corporate executives facing long hours, and even military units under high operational demand have experimented with modafinil.
While studies suggest it may improve executive function, memory, and sustained attention, concerns remain. Long-term safety in healthy populations is not fully established, and ethical questions about fairness and accessibility continue to spark debate.
Understanding Schedule IV Classification
What Schedule IV Means in U.S. Law
The Controlled Substances Act (CSA) places medications into five schedules based on their abuse potential and accepted medical use.
- Schedule I: High abuse potential, no accepted medical use (heroin, LSD).
- Schedule II: High abuse potential, accepted medical use (Adderall, Oxycodone).
- Schedule III: Moderate abuse potential (anabolic steroids, ketamine).
- Schedule IV: Low abuse potential relative to Schedule III (benzodiazepines, modafinil).
- Schedule V: Minimal abuse potential (cough preparations with codeine).
Thus, modafinil’s Schedule IV placement signals that while it is recognized as medically valuable, it still requires monitoring to prevent misuse.
Why Modafinil Was Classified Under Schedule IV
The DEA considered several factors when classifying modafinil:
- Clinical Evidence: Trials demonstrated medical benefits with relatively few side effects.
- Abuse Data: Reports showed low recreational use compared with other stimulants.
- Euphoric Potential: Modafinil does not produce a strong “high.”
- Dependence Risk: Withdrawal symptoms are rare and mild.
Taken together, these characteristics justify its Schedule IV classification, balancing access with safety.
Abuse Potential of Modafinil
While Provigil (modafinil) is widely regarded as safer than traditional stimulants, its Schedule IV classification acknowledges that abuse is possible. Understanding this risk requires examining clinical research, comparing modafinil to more tightly controlled stimulants, and reviewing real-world misuse patterns.
Evidence from Clinical Studies
Multiple studies have assessed modafinil’s abuse potential. Unlike amphetamines, which cause strong dopaminergic surges and rapid reinforcement, modafinil produces only a subtle elevation in dopamine levels.
- Laboratory studies: Participants given modafinil reported mild increases in alertness and focus but rarely experienced euphoria.
- Animal studies: Modafinil demonstrated weaker self-administration behaviors compared to cocaine or amphetamines.
- Human trials: Dependence and withdrawal symptoms were minimal, even with long-term therapeutic use.
The FDA evaluation concluded that while modafinil can be misused, the likelihood of dependence is substantially lower than Schedule II stimulants.
Comparison with Schedule II Stimulants
Schedule II stimulants such as Adderall and Ritalin are considered highly addictive due to their potent dopaminergic effects. When comparing modafinil against these drugs, several distinctions emerge:
| Feature | Modafinil (Provigil) | Amphetamines (Adderall) | Methylphenidate (Ritalin) |
|---|---|---|---|
| DEA Schedule | IV | II | II |
| Euphoric Effect | Low | High | Moderate |
| Dependence Risk | Low | High | Moderate to High |
| Withdrawal Symptoms | Mild to none | Fatigue, depression, irritability | Fatigue, mood swings |
| Recreational Popularity | Limited | High | High |
This comparison reinforces that modafinil is less reinforcing than its Schedule II counterparts, making it more suitable for long-term therapeutic use.
Patterns of Non-Medical Use
Although recreational use is relatively rare, some patterns of misuse have emerged:
- Academic and workplace use – Students and professionals sometimes take modafinil to extend study or work hours, believing it improves cognitive performance.
- Military applications – Military organizations in the U.S. and abroad have tested modafinil for sustaining wakefulness during extended missions.
- Recreational experimentation – A smaller group seeks modafinil for its subtle mood-lifting and focus-enhancing properties.
Unlike drugs such as cocaine or methamphetamine, modafinil does not produce a strong “rush,” which limits its attractiveness for recreational abuse. However, the social trend of using it as a “smart drug” continues to spark debate about long-term safety and ethics.
Benefits and Risks of Long-Term Modafinil Use
Modafinil’s profile is unique: it provides significant medical benefits but, like all drugs, comes with potential risks.
Cognitive Benefits
Clinical evidence suggests modafinil improves:
- Sustained attention
- Working memory
- Executive functioning
- Decision-making under fatigue
Healthy individuals taking modafinil often report enhanced productivity. However, the benefits are most pronounced in those experiencing sleep deprivation or fatigue-related deficits rather than fully rested individuals.
Psychiatric and Physical Side Effects
Long-term studies show modafinil is generally well tolerated, but some risks exist:
- Common side effects: Headache, nausea, nervousness, insomnia.
- Psychiatric risks: Rare cases of anxiety, mania, or hallucinations.
- Physical risks: Increased blood pressure and heart rate in sensitive populations.
- Dependence: Very low, but repeated off-label use can lead to psychological reliance.
Overall, the risk-to-benefit ratio favors its continued therapeutic use, especially when prescribed under proper medical supervision.
Regulatory Perspectives Across Countries
While the U.S. has placed modafinil under Schedule IV, regulations vary internationally.
United States
- Controlled as Schedule IV under the CSA.
- Requires a prescription.
- Insurance often covers approved conditions (narcolepsy, OSA, SWSD).
European Union
- Regulated differently across member states.
- In the UK, modafinil is prescription-only but not classified as a controlled drug.
- Some EU nations impose stricter controls due to concerns about cognitive enhancement misuse.
Asia and Emerging Markets
- In India, modafinil is widely available and less tightly controlled, leading to higher rates of non-prescription use.
- In Japan, regulations are much stricter, with limited availability.
- Emerging markets vary widely, often reflecting differences in public health policy and enforcement.
This global diversity in regulation highlights the ongoing debate: how to balance medical utility against the risk of misuse.
Patient Safety and Monitoring
The safe use of modafinil depends not only on its pharmacology but also on how it is prescribed and monitored in clinical practice. Physicians, pharmacists, and patients must work together to ensure that the therapeutic benefits outweigh potential risks.
Prescription Guidelines
When prescribing modafinil, physicians generally follow these recommendations:
- Start with the lowest effective dose – Typically 200 mg once daily in the morning.
- Avoid evening dosing – To prevent insomnia, modafinil should be taken early in the day.
- Assess comorbidities – Caution is advised in patients with cardiovascular disease, hypertension, or psychiatric conditions.
- Tapering not usually required – Unlike stronger stimulants, abrupt discontinuation rarely causes withdrawal, though fatigue may temporarily return.
These guidelines reflect modafinil’s safer pharmacological profile, but careful patient selection remains critical.
Screening for Misuse
Healthcare providers are encouraged to screen for potential misuse, especially in populations where off-label use is common. Red flags include:
- Patients requesting early refills.
- Reports of use for studying or productivity rather than sleep disorders.
- Online or “black market” sourcing.
Pharmacists also play a role in identifying potential diversion by monitoring prescription patterns.
Monitoring During Long-Term Use
For patients requiring extended therapy, regular follow-up is essential:
- Blood pressure checks to monitor cardiovascular effects.
- Mental health evaluations to detect rare psychiatric symptoms.
- Sleep studies to confirm ongoing need for treatment.
Through these measures, physicians can maintain the benefit-to-risk balance and ensure that patients use modafinil responsibly.
Ethical Considerations of Modafinil Use
The rise of modafinil as a cognitive enhancer has sparked intense ethical debates across medicine, academia, and the workplace. While its therapeutic value for narcolepsy and sleep disorders is clear, questions emerge when healthy individuals use it to gain an edge.
Workplace and Academic Implications
In universities and corporate environments, modafinil is sometimes seen as a “productivity booster.” This raises several concerns:
- Fairness – Is it ethical for some individuals to use modafinil for competitive advantage while others abstain?
- Pressure – Could workplaces or schools implicitly pressure individuals to use cognitive enhancers to keep up with peers?
- Safety – Long-term effects in healthy populations are not well studied, creating potential risks.
While some argue modafinil is simply another form of optimization (like caffeine), others caution that widespread off-label use blurs the line between therapy and enhancement.
Fairness and Access Issues
Another layer of complexity involves accessibility. If modafinil provides real cognitive benefits, will only the wealthy or privileged have consistent access? This could worsen inequalities in education and employment.
Bioethicists suggest that society must address these challenges before normalizing modafinil use outside medical contexts. Policies may need to balance individual freedom with collective fairness.
FAQ
1. Why is Provigil (modafinil) classified as Schedule IV?
Because it has recognized medical uses with low but real abuse potential. Unlike Schedule II stimulants, it produces less euphoria and carries a lower risk of dependence.
2. Is modafinil addictive?
Modafinil is considered to have a low risk of addiction. Most patients do not develop physical dependence, though some may develop psychological reliance if used off-label for productivity.
3. Can healthy people use modafinil safely?
While short-term studies suggest it is generally safe, long-term risks in healthy users are not well studied. Off-label use for cognitive enhancement remains controversial.
4. What conditions is modafinil FDA-approved to treat?
It is approved for narcolepsy, obstructive sleep apnea (OSA), and shift work sleep disorder (SWSD).
5. How does modafinil differ from Adderall or Ritalin?
Unlike these Schedule II stimulants, modafinil has a weaker dopaminergic effect, produces less euphoria, and has a much lower abuse potential.
6. Is modafinil available worldwide?
Yes, but regulations vary. In the U.S. it is Schedule IV, in the UK it is prescription-only but not controlled, and in some countries like India, it is more freely available.
Conclusion: Clinical Implications and Future Outlook
The Schedule IV classification of Provigil (modafinil) reflects its unique position in medicine. It offers profound benefits for patients with narcolepsy, OSA, and SWSD, while maintaining a relatively low risk of abuse compared to traditional stimulants.
As research continues, modafinil’s role may expand into new therapeutic areas, but careful monitoring, ethical consideration, and regulatory oversight will remain vital. The drug’s popularity as a cognitive enhancer underscores the need for balanced policies that preserve medical access while preventing misuse.
Ultimately, modafinil represents both promise and challenge a medication that enhances quality of life for those with sleep disorders, yet raises important questions when used outside its intended scope.
‼️ Disclaimer: The information provided in this article about modafinil is intended for informational purposes only and is not a substitute for professional medical consultation or recommendations. The author of the article are not responsible for any errors, omissions, or actions based on the information provided.
References:
- U.S. Food and Drug Administration. PROVIGIL. U.S. Department of Health and Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020717s037s038lbl.pdf . 2015
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006
- Willavize, S. A., Nichols, A. I., & Lee, J. Population pharmacokinetic modeling of armodafinil and its major metabolites. https://doi.org/10.1002/jcph.800 . 2016
- Fuxe K, et al. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005
- Mechanisms of modafinil: A review of current research. nih.gov. 2007
- PROVIGIL (modafinil) Tablets. FDA.GOV. 2010
- Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. https://doi.org/10.1186/s12883-021-02396-1 . 2021.
- Ciancio A, Moretti MC, Natale A, Rodolico A, Signorelli MS, Petralia A. Personality Traits and Fatigue in Multiple Sclerosis: A Narrative Review. Journal of Clinical Medicine. https://doi.org/10.3390/jcm12134518 . 2023
- Mereu, M., Bonci, A., Newman, A. H., & Tanda, G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. https://doi.org/10.1007/s00213-013-3232-4 . 2013
- Natsch, A. What makes us smell: The biochemistry of body odour and the design of new deodorant ingredients. CHIMIA International Journal for Chemistry. https://doi.org/10.2533/chimia.2015.414 . 2015
- Hamada, K., Haruyama, S., Yamaguchi, T., Yamamoto, K., Hiromasa, K., Yoshioka, M., Nishio, D., & Nakamura, M. What determines human body odour? Experimental Dermatology. https://doi.org/10.1111/exd.12380 . 2014