Introduction to Modafinil and Personalized Drug Response
What Is Modafinil?
Modafinil is often described as a “wakefulness-promoting agent,” but that label barely scratches the surface. Clinically, it is prescribed for conditions such as narcolepsy, obstructive sleep apnea, and shift work sleep disorder. Off-label, it has gained attention for cognitive enhancement, fatigue reduction, and improved alertness. Yet anyone who has discussed modafinil with others knows a curious truth: reactions vary wildly.
Why One Dose Does Not Fit All
Some people feel laser-focused on 100 mg, while others feel nothing until 200 mg or more. A subset experiences anxiety, headaches, or insomnia at doses that others tolerate effortlessly. This variability is not random. It is deeply rooted in biology, particularly genetics. That is where pharmacogenomics enters the conversation.
Understanding Pharmacogenomics
Definition and Core Principles
Pharmacogenomics is the study of how genetic variation influences drug response. In simple terms, your DNA helps determine how your body absorbs, metabolizes, distributes, and responds to medications. Think of a drug as a key and your biology as a lock. Small genetic differences can subtly reshape that lock.
Genes, Enzymes, and Drug Metabolism
Phase I and Phase II Metabolism
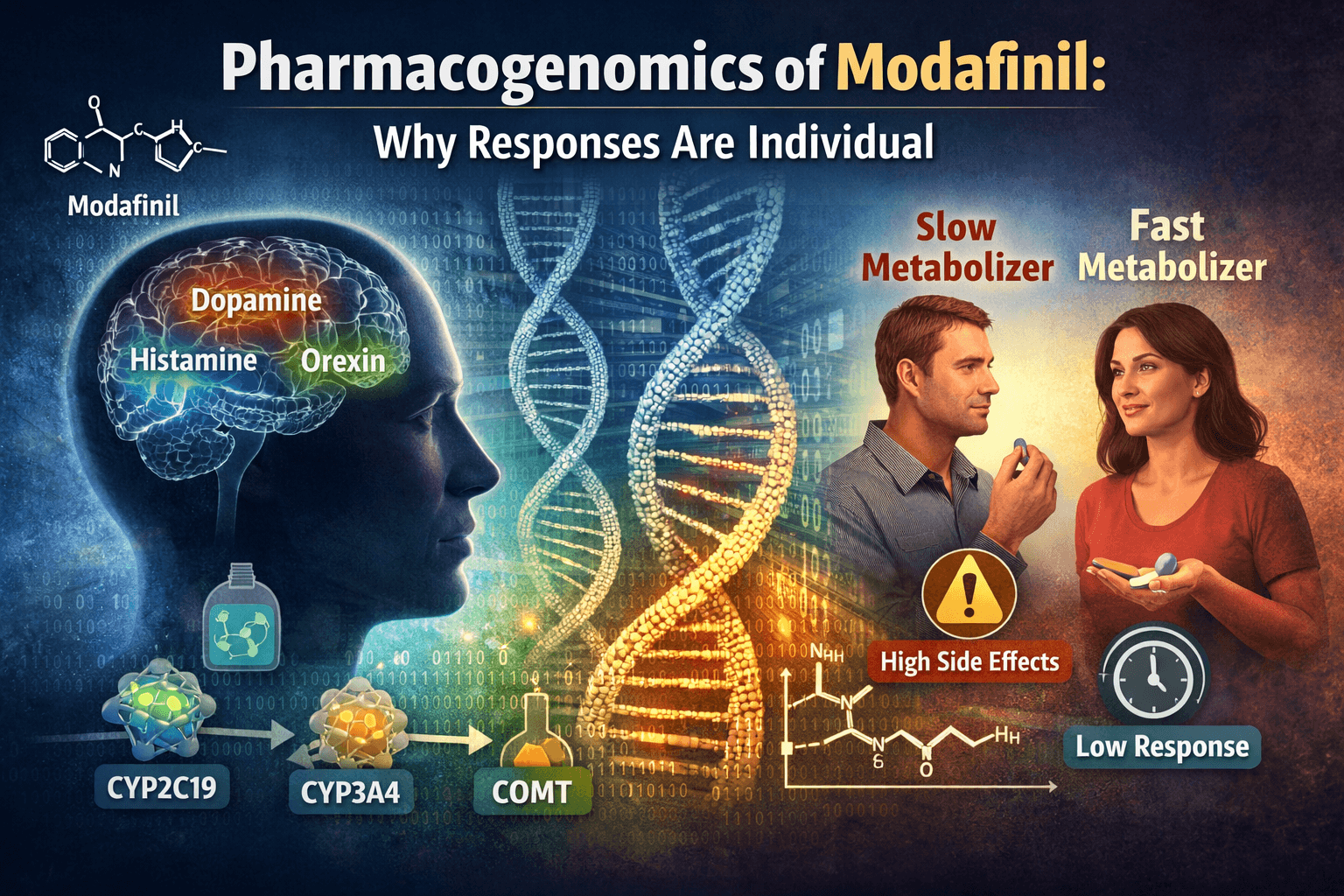
Most drugs, including modafinil, are processed by liver enzymes in two major stages. Phase I metabolism typically involves cytochrome P450 enzymes that modify the drug molecule. Phase II metabolism prepares it for elimination. Genetic variants in these enzymes can speed up or slow down these steps, dramatically altering blood concentrations of modafinil.
How Modafinil Works in the Brain
Neurochemical Pathways Involved
Unlike classic stimulants, modafinil has a complex mechanism of action. It influences multiple neurotransmitter systems, including dopamine, norepinephrine, histamine, orexin, and glutamate. This multi-target profile partly explains its nuanced effects.
Dopamine Transporter Interaction
One of the most important actions of modafinil is inhibition of the dopamine transporter. This increases extracellular dopamine, particularly in brain regions linked to motivation and attention. Genetic variation affecting dopamine signaling can therefore amplify or blunt modafinil’s effects.
Secondary Neurotransmitter Effects
Modafinil also indirectly boosts histamine and orexin activity, which promote wakefulness. Differences in receptor sensitivity and downstream signaling further contribute to individual variability.
Key Genes Influencing Modafinil Response
CYP3A4 and CYP2C19
Poor vs. Rapid Metabolizers
CYP3A4 and CYP2C19 are major enzymes involved in modafinil metabolism. Individuals with reduced-function variants may metabolize modafinil slowly, leading to higher plasma levels and increased risk of side effects. Rapid metabolizers, on the other hand, may clear the drug quickly and perceive it as weak or short-lived.
ABCB1 (P-glycoprotein Transporter)
ABCB1 encodes a transporter that pumps drugs out of the brain. Variants in this gene can alter how much modafinil actually reaches its central nervous system targets. Two people with identical blood levels may still experience different effects in the brain.
COMT and Dopamine Regulation
COMT influences dopamine breakdown, especially in the prefrontal cortex. Certain variants result in slower dopamine degradation, potentially intensifying modafinil’s cognitive effects or side effects such as anxiety.
Genetic Variability and Dosage Differences
Why Some People Need Higher Doses
Fast metabolism, efficient drug transport out of the brain, and lower receptor sensitivity can all reduce perceived efficacy. For these individuals, standard doses may feel underwhelming.
Why Others Experience Side Effects at Low Doses
Slow metabolism or heightened neurotransmitter sensitivity can lead to overstimulation even at modest doses. This is not intolerance in a psychological sense; it is predictable biology.
Ethnic and Population-Level Genetic Differences
Allele Frequency Variations
The prevalence of certain metabolic gene variants differs across populations. For example, reduced-function CYP2C19 variants are more common in some Asian populations than in European populations, influencing average dose requirements.
Clinical Implications Across Populations
These differences highlight why population-level dosing guidelines are approximations rather than precise instructions for individuals.
Age, Sex, and Epigenetic Factors
Hormonal Influences on Drug Metabolism
Sex hormones can modulate enzyme activity, which partly explains sex-based differences in drug response. Aging also alters liver function and enzyme expression.
Epigenetics and Gene Expression Changes
Gene expression is not static. Stress, diet, sleep patterns, and environmental exposures can switch genes on or off, subtly changing how modafinil is processed over time.
Drug–Drug Interactions and Genetic Risk
Enzyme Induction and Inhibition
Modafinil itself can induce or inhibit certain enzymes. When combined with other medications, genetically determined enzyme activity can magnify or mitigate these interactions.
Personalized Risk of Interactions
Two individuals taking the same drug combination may face very different risks due to genetic differences in metabolic capacity.
Modafinil Tolerance and Long-Term Use
Adaptive Neurobiology
With repeated use, the brain adapts. Receptor sensitivity and neurotransmitter release patterns may shift, influencing long-term efficacy.
Genetic Predisposition to Tolerance
Some genetic profiles may predispose individuals to faster neuroadaptation, making tolerance more likely.
Clinical Pharmacogenomic Testing
Available Genetic Tests
Commercial tests can identify variants in CYP enzymes, transporters, and neurotransmitter-related genes. These tests do not predict exact outcomes but provide probabilistic guidance.
Limitations and Current Barriers
Cost, limited clinician training, and incomplete evidence prevent widespread implementation, but the field is evolving rapidly.
Personalized Medicine and Modafinil
Precision Dosing Strategies
The future lies in tailoring dose and timing based on genetic, physiological, and lifestyle factors rather than population averages.
Future of Genotype-Guided Prescribing
As evidence grows, genotype-informed prescribing may become routine, reducing trial-and-error approaches.
Ethical and Regulatory Considerations
Genetic Privacy
Using genetic data raises legitimate concerns about privacy, consent, and data security.
Accessibility of Testing
Equitable access to pharmacogenomic tools remains a challenge that healthcare systems must address.
Practical Takeaways for Patients and Clinicians
Why Self-Comparison Is Misleading
Comparing doses or effects with others ignores genetic individuality. What works for one person may be inappropriate for another.
Communicating Variability Effectively
Clear communication about biological variability can reduce frustration and unrealistic expectations.
Conclusion
The varied responses to modafinil are not mysterious or subjective; they are rooted in genetics, neurobiology, and physiology. Pharmacogenomics provides a scientific framework for understanding why dosage and effects differ so dramatically among individuals. As personalized medicine advances, modafinil serves as a compelling example of why individualized treatment strategies are not a luxury but a necessity.
FAQ
1. Why does modafinil feel strong for some people and weak for others?
Because genetic differences affect metabolism, brain transport, and neurotransmitter sensitivity.
2. Can a genetic test tell me my ideal modafinil dose?
Not precisely, but it can indicate whether you are more likely to need higher or lower doses.
3. Does faster metabolism mean modafinil is ineffective?
No, it may simply require dose adjustment or timing optimization under medical guidance.
4. Are side effects always a sign of overdose?
Not necessarily. Genetic sensitivity can cause side effects even at low doses.
5. Will pharmacogenomics become standard in prescribing modafinil?
Trends suggest increasing adoption, though widespread clinical use will take time.
‼️ Disclaimer: The information provided in this article about modafinil is intended for informational purposes only and is not a substitute for professional medical consultation or recommendations. The author of the article are not responsible for any errors, omissions, or actions based on the information provided.
References:
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006
- McClellan, K. J., & Spencer, C. M. Modafinil: A review of its pharmacology and clinical efficacy in the management of narcolepsy. CNS Drugs, 311–324. https://doi.org/10.2165/00023210-199809040-00006 . 1998.
- Willavize, S. A., Nichols, A. I., & Lee, J. Population pharmacokinetic modeling of armodafinil and its major metabolites. https://doi.org/10.1002/jcph.800 . 2016
- U.S. Food and Drug Administration. PROVIGIL. U.S. Department of Health and Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020717s037s038lbl.pdf . 2015
- Gilleen, J., Michalopoulou, P. G., Reichenberg, A., Drake, R., Wykes, T., Lewis, S. W., & Kapur, S. Modafinil combined with cognitive training is associated with improved learning in healthy volunteers a randomised controlled trial. European Neuropsychopharmacology. 529–539. https://doi.org/10.1016/j.euroneuro.2014.01.001 . 2014
- Greenblatt, K., Adams, N. Modafinil. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK531476/ . 2025
- Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. https://doi.org/10.1186/s12883-021-02396-1 . 2021.
- Mereu, M., Bonci, A., Newman, A. H., & Tanda, G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. https://doi.org/10.1007/s00213-013-3232-4 . 2013
- Ciancio A, Moretti MC, Natale A, Rodolico A, Signorelli MS, Petralia A. Personality Traits and Fatigue in Multiple Sclerosis: A Narrative Review. Journal of Clinical Medicine. https://doi.org/10.3390/jcm12134518 . 2023
- Natsch, A. What makes us smell: The biochemistry of body odour and the design of new deodorant ingredients. CHIMIA International Journal for Chemistry. https://doi.org/10.2533/chimia.2015.414 . 2015