Last Updated on 11/02/2026 by James Anderson
Beyond Wakefulness: A Molecular Perspective
Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) is widely recognized as a first-line wakefulness-promoting agent (eugeroic) for narcolepsy, shift work sleep disorder, and residual sleepiness in obstructive sleep apnea. However, its classification as a simple “alertness pill” dramatically undersells its sophisticated and multi-faceted biochemical fingerprint.
Unlike classical stimulants (amphetamines, methylphenidate) that induce broad, high-amplitude monoamine release, Modafinil operates through a subtle, region-specific, and multi-transmitter modulation of key neural circuits. This results in a unique clinical profile: cognitive enhancement without euphoria, wakefulness without significant sympathomimetic overdrive, and low abuse potential. This review provides an in-depth, evidence-based analysis of the biochemical, cellular, and network-level effects of Modafinil on the mammalian brain, synthesizing data from molecular pharmacology, neuroimaging, and behavioral neuroscience.
Primary Mechanism: Dopamine Transporter (DAT) Inhibition and Beyond
1. The DAT-Centric Model
The most established and quantitatively significant mechanism of Modafinil is competitive inhibition of the dopamine transporter (DAT) . By binding to DAT, Modafinil blocks the reuptake of dopamine (DA) from the synaptic cleft into presynaptic terminals.
- Regional Specificity: Unlike cocaine or amphetamines, Modafinil’s DAT occupancy is not uniform. PET studies in humans using [¹¹C]cocaine and [¹¹C]raclopride demonstrate that clinically relevant doses (200-400 mg) achieve approximately 50% DAT occupancy in the striatum and nucleus accumbens. This partial occupancy is critical; it elevates extracellular DA to pro-cognitive levels without the massive, rapid surges that produce euphoria and drive addiction.
- Functional Consequence: Increased tonic DA levels in the prefrontal cortex (PFC) and striatum enhance signal-to-noise ratio in cortico-striatal-thalamic loops, optimizing executive function, working memory, and motivation.
2. The Norepinephrine Connection
Modafinil also inhibits the norepinephrine transporter (NET) , though with lower potency than DAT.
- Locus Coeruleus Activation: By elevating extracellular norepinephrine (NE) in the cortex and hypothalamus, Modafinil enhances the “alerting” and “orienting” networks of attention. This NE surge is largely responsible for the subjective feeling of focused vigilance and improved reaction time.
3. Why Modafinil is Not Addictive: The Kinetic Hypothesis
The key to Modafinil’s safety lies in its slow DAT occupancy kinetics and lack of effect on the vesicular monoamine transporter (VMAT). Amphetamines actively reverse VMAT, forcing DA into the synapse. Modafinil does not. Consequently, DA levels rise slowly and remain within a physiological range, avoiding the rapid, supraphysiological spikes that condition addictive behavior.
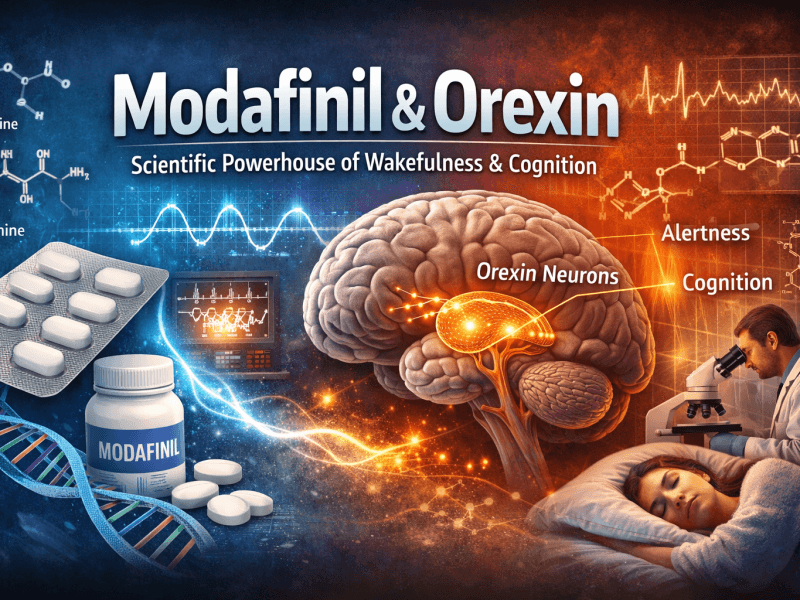
The Orexin (Hypocretin) and Histamine Axis: Master Regulators of Arousal
A defining feature of Modafinil’s pharmacology is its engagement of the brain’s endogenous wakefulness circuitry.
1. Orexin Neuron Activation
Orexin (hypocretin) neurons, located exclusively in the lateral hypothalamus, project throughout the brain to stabilize wakefulness. Modafinil has been shown to directly activate orexin neurons, likely via indirect glutamatergic mechanisms.
- Functional Impact: Orexin release reinforces arousal, increases locomotor activity, and promotes motivated behavior. This explains why Modafinil effectively combats the pathological sleepiness of narcolepsy, a disorder characterized by orexin neuron degeneration.
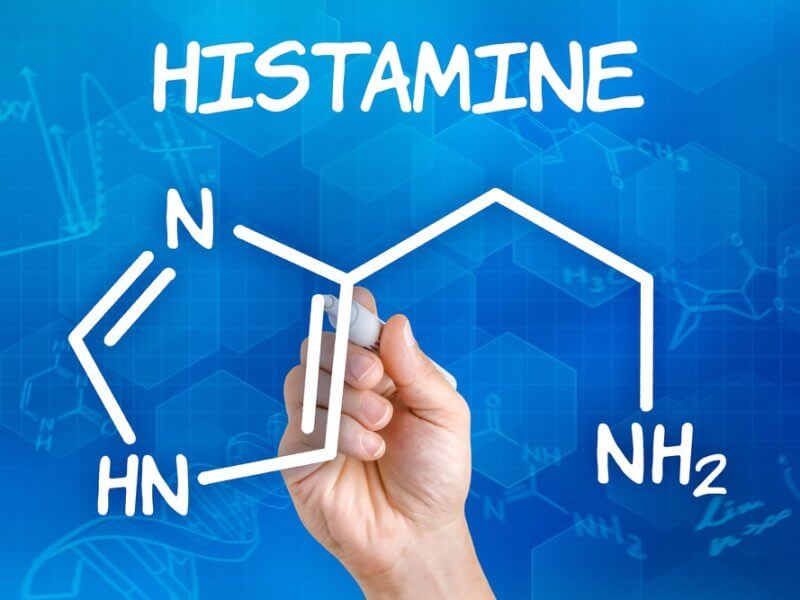
2. Histaminergic Surge
Activated orexin neurons stimulate the tuberomammillary nucleus (TMN) , the brain’s sole source of histamine.
- Mechanism: Modafinil increases histamine release in the cortex and hypothalamus. Histamine is a potent wake-promoting neurotransmitter; H1 receptor antagonists cause profound sedation. This histaminergic surge is a primary downstream effector of Modafinil’s pro-arousal effects.
Modulation of Glutamate and GABA: Rebalancing Excitation and Inhibition
Modafinil exerts a unique bidirectional modulation of the brain’s primary excitatory (glutamate) and inhibitory (GABA) neurotransmitters.
1. Enhanced Glutamatergic Transmission
- Mechanism: Microdialysis studies in rodents demonstrate that Modafinil significantly increases extracellular glutamate in the hippocampus, striatum, and prefrontal cortex. This is not due to excitotoxicity but rather enhanced physiologic release.
- Synaptic Plasticity: Elevated glutamate enhances long-term potentiation (LTP) , the cellular correlate of learning and memory. This mechanism underlies Modafinil’s ability to improve performance on cognitive tasks requiring synaptic adaptation.
2. Reduced GABAergic Tone
- Mechanism: Conversely, Modafinil decreases GABA release in the cortex and hypothalamus. By reducing inhibitory tone, the brain’s excitatory networks are more readily recruited for cognitive tasks.
- Net Effect: This push-pull dynamic increasing excitation while decreasing inhibition shifts the cortical network into a state of “optimized gain,” facilitating rapid information processing and attentional focus.
Neuroprotection: Antioxidant and Anti-Inflammatory Effects
A lesser-known but clinically significant biochemical effect of Modafinil is its neuroprotective capacity, which extends beyond its acute cognitive actions.
1. Oxidative Stress Mitigation
Modafinil upregulates key endogenous antioxidant enzymes:
- Superoxide Dismutase (SOD): Converts superoxide radicals to hydrogen peroxide.
- Catalase (CAT): Decomposes hydrogen peroxide to water.
- Glutathione Peroxidase (GPx): Reduces lipid hydroperoxides.
These effects have been demonstrated in both neuronal cell cultures and animal models of neurotoxicity. By reducing lipid peroxidation and protein carbonylation, Modafinil preserves membrane integrity and mitochondrial function.
2. Anti-Inflammatory Cytokine Modulation
Chronic neuroinflammation is a hallmark of neurodegenerative disease. Modafinil has been shown to suppress the production of pro-inflammatory cytokines:
- Tumor Necrosis Factor-alpha (TNF-α)
- Interleukin-6 (IL-6)
- Interleukin-1 beta (IL-1β)
This anti-inflammatory action may contribute to its potential therapeutic utility in conditions like multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease, where neuroinflammation drives pathology.
3. Mitochondrial Energetics
Proteomic analyses reveal that Modafinil upregulates proteins involved in oxidative phosphorylation (Complex I-V) and the tricarboxylic acid (TCA) cycle. By enhancing neuronal energy metabolism, Modafinil may increase the brain’s resilience to metabolic stress and excitotoxicity.
Comparative Neurochemistry: Modafinil vs. Classical Stimulants
Understanding Modafinil’s unique profile requires direct comparison with other prototypic agents.
| Parameter | Modafinil | Amphetamine | Methylphenidate |
|---|---|---|---|
| Primary Mechanism | DAT/NET inhibition | DAT/NET reversal & release | DAT/NET inhibition |
| VMAT Interaction | None | Releases vesicular DA | None |
| DAT Occupancy (Therapeutic) | ~50% | High (>80%) | High (>60-70%) |
| DA Elevation Kinetics | Slow, sustained | Rapid, high-amplitude | Moderate |
| Orexin/Histamine Activation | Yes | Minimal | Minimal |
| Glutamate/GABA Modulation | Yes | Minimal | Minimal |
| Abuse Potential (Schedule) | Low (IV) | High (II) | High (II) |
| Neurotoxicity Risk | Very low | Present at high doses | Low |
| Cognitive Enhancement (Sleep-Deprived) | +++ | ++ | ++ |
| Euphoria | Minimal/Absent | +++ | ++ |
Key Insight: Modafinil is not simply a “weak stimulant.” It is a pharmacologically distinct entity that achieves cognitive enhancement through a fundamentally different neural systems approach.
Advanced Neuroimaging and Systems-Level Effects
Modern neuroscience has moved beyond measuring single transmitters to mapping large-scale brain networks. Modafinil produces consistent, replicable effects on functional connectivity.
1. fMRI Findings
- Prefrontal Cortex (PFC): Modafinil increases task-related activation in the dorsolateral PFC (DLPFC) and anterior cingulate cortex (ACC) during working memory and attentional tasks.
- Default Mode Network (DMN): The DMN is associated with mind-wandering and self-referential thought. Modafinil suppresses DMN activity while enhancing activation in task-positive networks. This “network antagonism” is a neural correlate of improved sustained attention.
- Functional Connectivity: Resting-state fMRI reveals Modafinil increases connectivity between the ACC and striatum, integrating cognitive control and reward circuits.
2. PET and Molecular Imaging
- Dopamine Transporter Occupancy: Confirms ~50% striatal DAT occupancy at 200 mg.
- Cerebral Glucose Metabolism: Modafinil increases the cerebral metabolic rate of glucose (CMRglu) in cortical regions, indicating enhanced neuronal activity and energy utilization.
Long-Term Neuroadaptation and Safety
A critical question for any CNS-active agent is whether chronic use induces harmful neuroadaptation.
- Absence of Dopamine D₂ Receptor Downregulation: Unlike chronic amphetamine use, which downregulates D₂ receptors (a marker of addiction vulnerability), studies in rats and non-human primates show that prolonged Modafinil administration does not significantly alter D₂ receptor density or sensitivity.
- Hippocampal Neurogenesis: Contrary to the suppressive effects of stress and some stimulants, one study (Brandt et al., 2014) reported that Modafinil increased adult hippocampal neurogenesis in rodents, suggesting potential pro-plasticity effects.
- Tolerance: Tolerance to Modafinil’s subjective effects can develop in some users, but it is less pronounced and slower to develop than with amphetamines. Drug holidays are often recommended to maintain efficacy.
FAQ
How quickly does Modafinil’s biochemical action translate to cognitive effects?
Modafinil is rapidly absorbed, reaching peak plasma concentrations (Tmax) in 2-4 hours. DAT occupancy and downstream neurotransmitter changes occur within this window, with subjective alertness typically noted within 30-90 minutes of ingestion.
Does Modafinil cause neurotoxicity at high or chronic doses?
Current evidence suggests no. Unlike amphetamines, which can cause dopamine terminal damage at high, repeated doses, Modafinil has not demonstrated significant neurotoxicity in preclinical models. Its antioxidant properties may even be neuroprotective. However, long-term human data is still limited.
Can Modafinil enhance creativity or just focus?
This is nuanced. Modafinil robustly enhances convergent thinking (focused, analytical problem-solving). Its effects on divergent thinking (creativity, idea generation) are mixed; some studies show no improvement or even a slight reduction, possibly because it reduces mind-wandering, which is sometimes linked to creative insight.
Why doesn’t Modafinil work for some people?
Genetic polymorphisms in the DAT1 (SLC6A3) and COMT genes can influence response. Additionally, individuals with high baseline alertness may experience minimal subjective effects. Lack of effect can also be due to poor sleep hygiene, tolerance, or misdiagnosis of the underlying condition.
Is Modafinil’s mechanism similar to the “Limitless” pill?
No. The fictional NZT-48 in Limitless is portrayed as unlocking 100% of the brain’s unused capacity a biological impossibility. Modafinil does not create “superhuman” intelligence. It optimizes existing neural circuits, particularly under conditions of fatigue or impairment, providing a subtle, state-dependent enhancement of cognitive efficiency.
Conclusion: A Unique Molecular Signature
The biochemical effects of Modafinil on the brain represent a sophisticated and multi-dimensional pharmacology that defies simple categorization. It is not a traditional stimulant, not a pure nootropic, and not merely a wakefulness agent. Instead, Modafinil occupies a unique pharmacological niche defined by:
- Targeted DAT inhibition with slow, partial occupancy.
- Engagement of the orexin-histamine arousal axis.
- Bidirectional glutamate/GABA modulation favoring excitation.
- Demonstrated neuroprotective and antioxidant properties.
- A favorable long-term neuroadaptation profile.
This distinct biochemical signature explains its clinical utility beyond sleep disorders, its favorable side effect profile, and its low abuse liability. As molecular and neuroimaging techniques continue to advance, our understanding of how this remarkable compound modulates the human brain will only deepen, potentially unlocking new therapeutic applications in neuropsychiatry and neurorehabilitation.
For clinicians and researchers: Appreciating the nuance of Modafinil’s mechanism is essential for rational prescribing and for guiding patients toward realistic expectations about what this medication can and cannot achieve.
‼️ Disclaimer: The information provided in this article about modafinil is intended for informational purposes only and is not a substitute for professional medical consultation or recommendations. The author of the articl eare not responsible for any errors, omissions, or actions based on the information provided.
References:
- Aron, A. R., Robbins, T. W., and Poldrack, R. A. Inhibition and the right inferior frontal cortex. 2004
- Badre, D., and Wagner, A. D. Frontal lobe mechanisms that resolve proactive interference. Cereb. 2005
- Battleday, R. M., Brem. Modafinil for cognitive neuroenhancement in healthy non sleep deprived subjects: a systematic review. 2015
- Boly, M., Balteau, E., Schnakers, C., Degueldre, C., Moonen, G., Luxen, A. Baseline brain activity fluctuations predict somatosensory perception in humans. 2007
- Brandt, M. D., Ellwardt, E., and Storch, A.Short and long term treatment with modafinil differentially affects adult hippocampal neurogenesis. 2014
- Cera, N., Tartaro, A., and Sensi, S. L. Modafinil alters intrinsic functional connectivity of the right posterior insula: a pharmacological resting state fMRI study. 2014
- Dance, A. Smart drugs: a dose of intelligence. Nature. 2016
- Dawson, N., Thompson, R. J., McVie, A., Thomson, D. M., Morris, B. J., and Pratt, J. A. Modafinil reverses phencyclidine induced deficits in cognitive flexibility, cerebral metabolism, and functional brain connectivity. 2016
- Desmond, J. E. A meta analysis of cerebellar contributions to higher cognition from PET and fMRI studies. 2012