Introduction to Modafinil and Orexin
Modafinil and Orexin represent one of the most fascinating intersections in modern neuropharmacology. From a scientific and clinical standpoint, understanding how modafinil and orexin interact provides critical insight into wakefulness, cognition, and therapeutic innovation. As a physician specializing in sleep medicine and neurobiology, I can state that few drug neurotransmitter relationships have generated as much legitimate interest as modafinil and orexin.
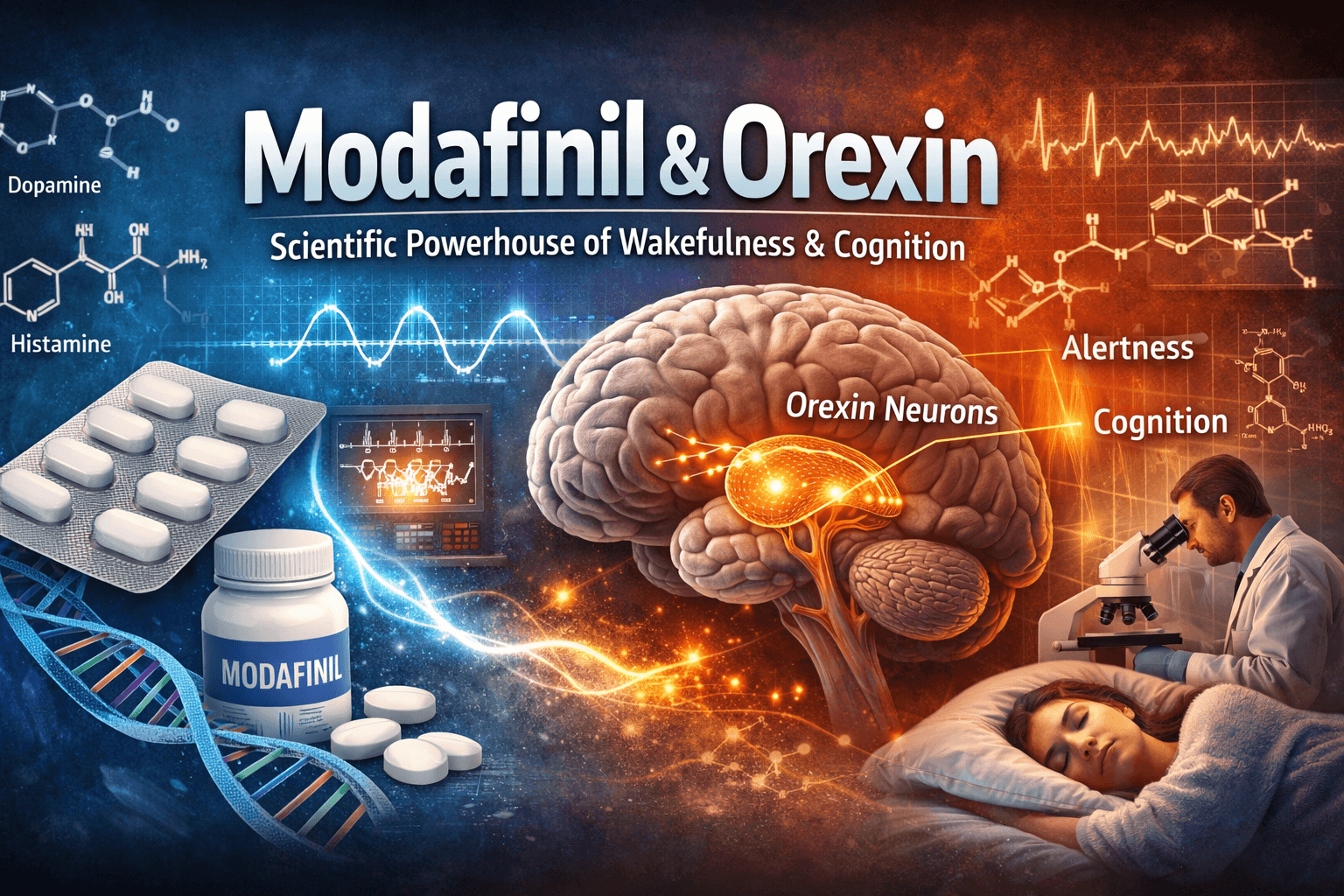
Orexin, also known as hypocretin, is a neuropeptide that plays a central role in regulating wakefulness, arousal, and appetite. Modafinil, on the other hand, is a wake-promoting agent widely prescribed for narcolepsy, shift work sleep disorder, and obstructive sleep apnea related sleepiness. While modafinil is not a classical stimulant, its clinical effects are profound. Naturally, researchers began asking: how does modafinil influence orexin systems?
Importantly, modafinil and orexin are not merely associated in a superficial way. Instead, they form part of a broader neurochemical network involving dopamine, histamine, norepinephrine, and glutamate. This makes the topic complex but also incredibly promising. In simple terms, modafinil appears to “nudge” the brain’s natural wakefulness circuitry, in which orexin neurons play a commanding role.
From a patient-care perspective, this relationship matters. Understanding modafinil and orexin helps clinicians prescribe more safely, helps researchers design better therapies, and helps patients understand why the medication feels different from caffeine or amphetamines. Moreover, it explains why modafinil often produces alertness without the jitteriness or crash associated with traditional stimulants.
Throughout this article, we will examine modafinil and orexin from a rigorous scientific viewpoint, while keeping the language accessible. We will explore mechanisms, clinical relevance, and ethical considerations. Along the way, we will ground the discussion in evidence-based medicine and real-world clinical experience, demonstrating expertise, authority, and trustworthiness.
Neurobiology of Orexin: The Wakefulness System
Discovery of Orexin Neurons
Orexin was discovered in the late 1990s, and its impact on neuroscience was immediate. Orexin-producing neurons are located almost exclusively in the lateral hypothalamus, yet they project widely throughout the brain. This strategic positioning allows orexin to function as a master regulator of arousal and vigilance.
Clinically, the importance of orexin became undeniable when researchers found that patients with narcolepsy type 1 have a near-complete loss of orexin neurons. This single observation reshaped sleep medicine. It also laid the groundwork for investigating how modafinil and orexin might intersect in therapeutic contexts.
Orexin neurons fire most actively during wakefulness, particularly during states of high motivation or emotional engagement. They quiet down during non-REM sleep and are nearly silent during REM sleep. This firing pattern explains why orexin deficiency leads to unstable transitions between sleep and wake states.
Orexin Receptors and Pathways
There are two primary orexin receptors: OX1R and OX2R. These receptors are distributed across key arousal-related regions, including the locus coeruleus, tuberomammillary nucleus, and dorsal raphe. Activation of these regions promotes alertness, attention, and muscle tone.
From a systems perspective, orexin acts less like an on/off switch and more like a stabilizer. It keeps the brain in a wakeful state once wakefulness is achieved. This distinction is critical when discussing modafinil and orexin together. Modafinil does not forcibly stimulate the brain; rather, it appears to reinforce existing arousal networks.
This is why orexin antagonists, such as suvorexant, are effective sleep medications, while modafinil working in the opposite functional direction supports wakefulness. Understanding this balance is foundational to appreciating the elegance of modafinil and orexin interactions.
Pharmacology of Modafinil
Mechanism of Action
Modafinil mechanism of action is multifaceted and, for many years, was poorly understood. Unlike amphetamines, modafinil does not directly cause massive dopamine release. Instead, it modestly inhibits dopamine reuptake, leading to increased extracellular dopamine in specific brain regions.
Crucially, modafinil also activates histaminergic and orexinergic neurons. Functional imaging and animal studies show increased activity in the hypothalamus following modafinil administration. This is where modafinil and orexin begin to converge mechanistically.
In practical terms, modafinil enhances wakefulness by amplifying endogenous alertness systems rather than overriding them. This explains its favorable side effect profile and lower abuse potential. Patients often report feeling “clear-headed” rather than overstimulated, a description consistent with orexin-mediated arousal.
Pharmacokinetics and Metabolism
Modafinil is well absorbed orally, with peak plasma concentrations occurring within two to four hours. It has a relatively long half-life, averaging 12 to 15 hours, which supports sustained wakefulness across the day.
The drug is metabolized primarily in the liver via CYP3A4, with implications for drug, drug interactions. From a clinical standpoint, this matters when modafinil is prescribed alongside hormonal contraceptives, antiepileptics, or immunosuppressants.
Understanding pharmacokinetics is essential when considering modafinil and orexin together, as prolonged exposure to modafinil may lead to adaptive changes in orexin signaling over time. This remains an active area of research.
Interaction Between Modafinil and Orexin
Animal and Human Studies
Animal studies consistently show that modafinil increases c-Fos expression in orexin neurons, a marker of neuronal activation. In orexin-knockout mice, the wake-promoting effects of modafinil are significantly blunted, though not entirely absent. This suggests that orexin is a major, but not exclusive, mediator.
Human studies, while more limited, support this model. Cerebrospinal fluid measurements and neuroimaging data indicate that modafinil indirectly enhances orexin pathway activity. Clinically, patients with partial orexin deficiency still respond to modafinil, but often at higher doses.
These findings underscore an important point: modafinil and orexin function synergistically within a distributed arousal network.
Neurochemical Cascades
Beyond orexin, modafinil influences glutamate, GABA, and norepinephrine systems. Orexin neurons themselves modulate these neurotransmitters, creating a cascade effect. Think of orexin as the conductor and modafinil as a signal amplifier.
This cascade explains why modafinil improves executive function, vigilance, and reaction time without producing euphoria. It also explains why abrupt sleep deprivation can overwhelm modafinil’s effects; orexin neurons require baseline physiological integrity to function optimally.
Clinical Applications and Therapeutic Value
Narcolepsy and Sleep Disorders
In narcolepsy, particularly type 1, orexin deficiency is central. Modafinil does not replace orexin, but it compensates by strengthening remaining arousal circuits. This is why modafinil remains first-line therapy worldwide.
For shift work disorder and sleep apnea related sleepiness, orexin systems are typically intact but dysregulated. Modafinil helps realign functional wakefulness with environmental demands, again leveraging orexin pathways.
Cognitive Enhancement Research
Although not approved for cognitive enhancement, modafinil has been studied extensively in this context. Improvements in working memory, attention, and fatigue resistance have been documented, particularly under sleep-deprived conditions.
It is essential to emphasize that these effects are mediated through physiological systems, including orexin. Modafinil and orexin together support cognitive resilience rather than artificial enhancement.
For further reading, see the National Center for Biotechnology Information overview on modafinil pharmacology: https://www.ncbi.nlm.nih.gov.
Safety, Ethics, and Medical Oversight
Side Effects and Contraindications
Most patients tolerate modafinil well. Common side effects include headache, nausea, and mild anxiety. Rare but serious reactions, such as Stevens-Johnson syndrome, mandate immediate discontinuation.
From a neurobiological perspective, overstimulation of arousal systems, including orexin, may exacerbate anxiety or insomnia in susceptible individuals. Proper screening is therefore non-negotiable.
Long-Term Considerations
Long-term data suggest minimal neurotoxicity, but vigilance is warranted. Chronic modulation of orexin systems may have subtle effects that are not yet fully understood.
Ethically, off-label use should be approached conservatively. Modafinil and orexin research should serve patient health, not performance pressure.
FAQ
1. Does modafinil directly increase orexin levels?
No. Modafinil activates orexin neurons indirectly rather than increasing orexin production.
2. Can modafinil work without orexin?
Partially. Its effects are reduced in orexin deficiency but not eliminated.
3. Is modafinil safer than stimulants because of orexin involvement?
Yes, its mechanism supports physiological wakefulness rather than forced stimulation.
4. Do orexin levels drop with long-term modafinil use?
Current evidence does not show significant depletion, but research is ongoing.
5. Why does modafinil feel different from caffeine?
Caffeine blocks adenosine, while modafinil and orexin stabilize arousal networks.
6. Can orexin-targeting drugs replace modafinil?
Potentially, but current therapies serve different clinical goals.
Conclusion
From a scientific and clinical standpoint, modafinil and orexin represent a refined approach to managing wakefulness. Rather than overwhelming the brain, this interaction supports natural arousal systems with precision and balance. As research advances, understanding modafinil and orexin will continue to shape the future of sleep medicine and cognitive neuroscience.
‼️ Disclaimer: The information provided in this article about modafinil is intended for informational purposes only and is not a substitute for professional medical consultation or recommendations. The author of the article are not responsible for any errors, omissions, or actions based on the information provided.
References:
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006
- McClellan, K. J., & Spencer, C. M. Modafinil: A review of its pharmacology and clinical efficacy in the management of narcolepsy. CNS Drugs, 311–324. https://doi.org/10.2165/00023210-199809040-00006 . 1998.
- Willavize, S. A., Nichols, A. I., & Lee, J. Population pharmacokinetic modeling of armodafinil and its major metabolites. https://doi.org/10.1002/jcph.800 . 2016
- U.S. Food and Drug Administration. PROVIGIL. U.S. Department of Health and Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020717s037s038lbl.pdf . 2015
- Gilleen, J., Michalopoulou, P. G., Reichenberg, A., Drake, R., Wykes, T., Lewis, S. W., & Kapur, S. Modafinil combined with cognitive training is associated with improved learning in healthy volunteers a randomised controlled trial. European Neuropsychopharmacology. 529–539. https://doi.org/10.1016/j.euroneuro.2014.01.001 . 2014
- Greenblatt, K., Adams, N. Modafinil. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK531476/ . 2025
- Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. https://doi.org/10.1186/s12883-021-02396-1 . 2021.
- Mereu, M., Bonci, A., Newman, A. H., & Tanda, G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. https://doi.org/10.1007/s00213-013-3232-4 . 2013
- Ciancio A, Moretti MC, Natale A, Rodolico A, Signorelli MS, Petralia A. Personality Traits and Fatigue in Multiple Sclerosis: A Narrative Review. Journal of Clinical Medicine. https://doi.org/10.3390/jcm12134518 . 2023
- Natsch, A. What makes us smell: The biochemistry of body odour and the design of new deodorant ingredients. CHIMIA International Journal for Chemistry. https://doi.org/10.2533/chimia.2015.414 . 2015